Mechanism of Action
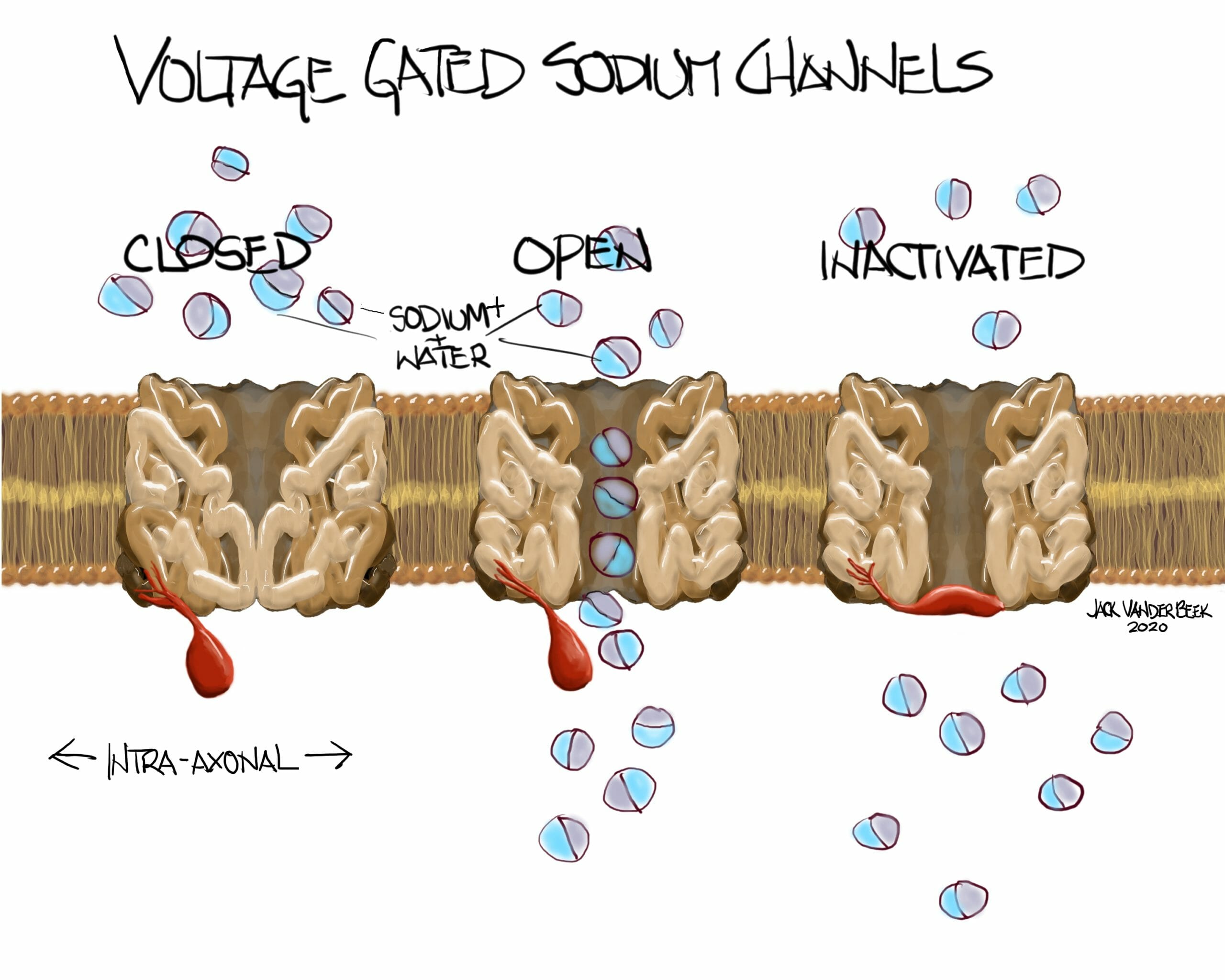
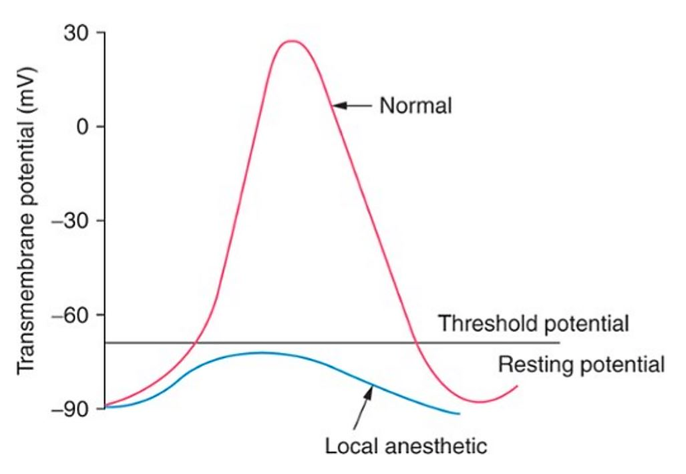
- The nerve cell typically has a negative resting membrane potential of -70 mV. When the neuron is stimulated at a sufficient intensity, a small influx of sodium ions enters the axonal membrane until a threshold potential is achieved, resulting in an Action Potential and depolarization of the neuron which propagates an electrical signal (saltatory conduction) along a nerve fiber to stimulate a muscle or sensory response.
- A stimulus needs to be applied at an adequate strength level and for a sufficient amount of time to elicit an Action Potential response.
- In a normal functioning sodium voltage-gated channel, a conformational change from the closed state to the activated state occurs during the excitation and depolarization phase when a threshold potential is achieved. Following depolarization, the channel transitions back into an inactivated and closed states facilitating the transmission of additional, subsequent stimuli.
- Local anesthetics produce a reversible blockade of impulses conducted along neural pathways by attaching to the inactivated and open states on voltage-gated, sodium channels in the axonal membrane. This binding prevents the sodium voltage-gated channel from undergoing a conformational change, and thus inhibits sodium ion influx into the axoplasm and depolarization of the nerve fiber.
Depolarization and Binding of Sodium Voltage Gated Channel
- There is a positive association between the rate of depolarization and peak amplitude of a nerve’s action potential and the binding capacity of local anesthetics to the nerve’s sodium channels.
- Therefore, successive activations increase the number of bound sodium channels as 1) more binding sites are available during activation (excitation and depolarization) and 2) binding site dissociation with local anesthetics is slower from previously inactivated channels than from channels in the resting state
- The previous two points help us understand why local anesthetics preferentially block fibers with faster conduction velocities, such as small myelinated A gamma motor and A delta sensory axons more than large myelinated axons (A alpha and beta), and least of all, the slow and small, nonmyelinated C fibers.
Deposition and Clearance of Local Anesthetics
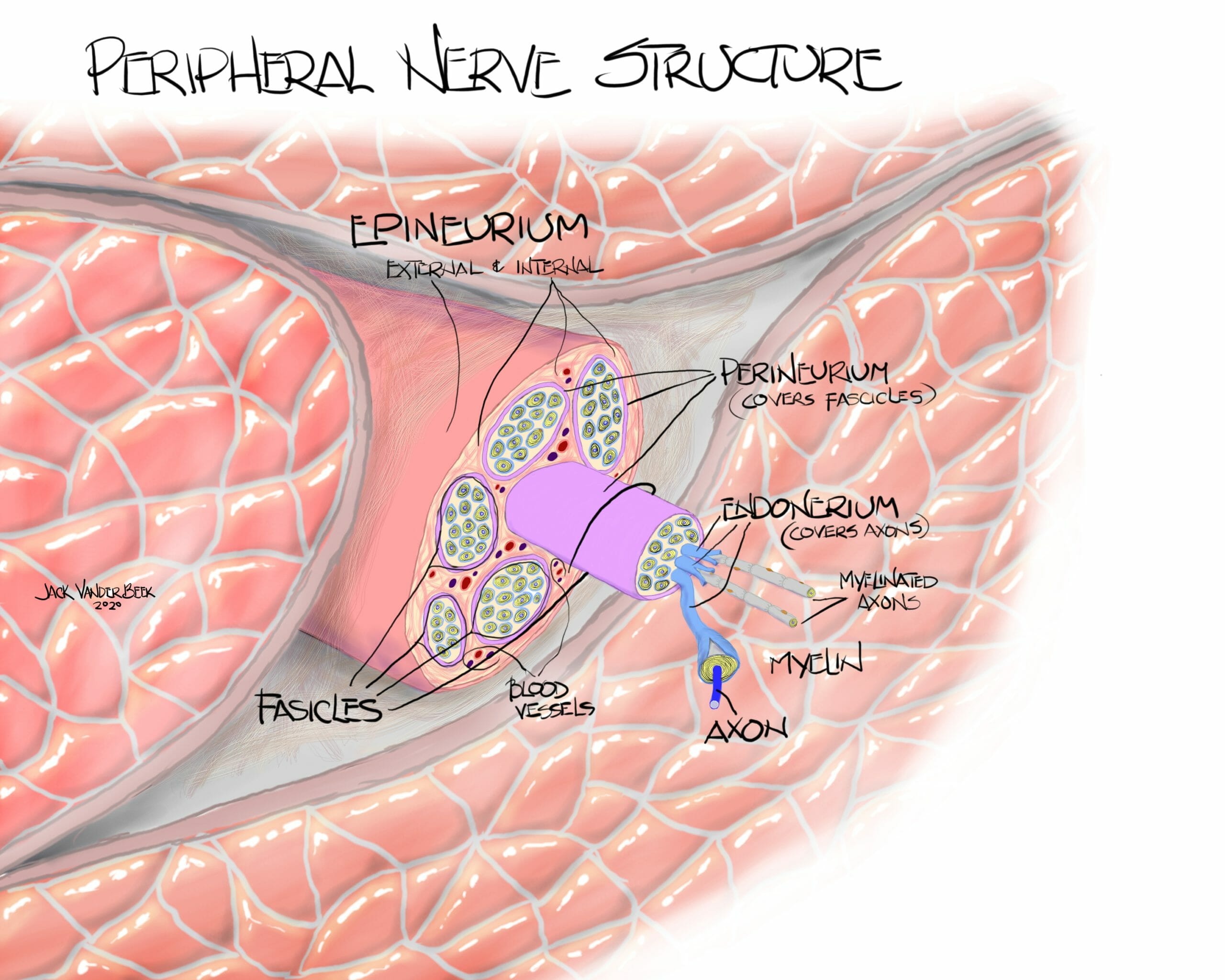
- Local anesthetics are placed in proximity to a nerve in area labeled as the external epineurium and inside the neural sheath.
- Local anesthetics placed within a nerve fascicle can lead to an intrafascicular rupture and nerve injury.
- Some amount of the local anesthetic is rendered inconsequential due to protein binding, absorption into the systemic circulation, or in the case of the esters, metabolized via a hydrolysis reaction.
- The remainder of the local anesthetic is available to penetrate the axonal membrane and commence blockade.
- The onset and duration of blockade depends on many factors including the local anesthetic’s lipid solubility and pKa.
- A greater concentration of local anesthetic predictably produces a greater depression of electrical impulses.
Physicochemical Properties
- All local anesthetics are weak bases.
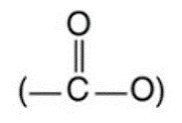
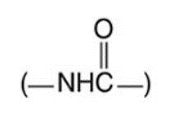
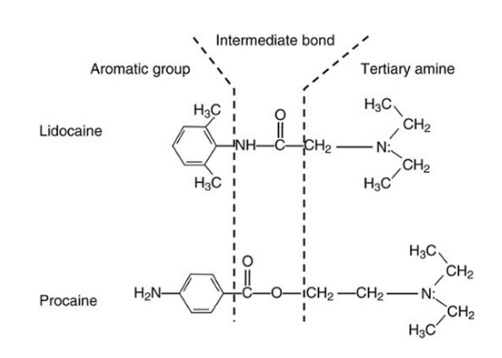
- All local anesthetics contain an aromatic ring, an intermediate chain (either an ester or an amide), and a tertiary amine end.
- The aromatic ring end imparts lipophilic properties.
- The tertiary amine end is hydrophilic, as it carries a positive charge (can accept protons to become “protonated”).
- Most local anesthetics are formulated as hydrochloride salts to lengthen shelf life and optimize solubility.
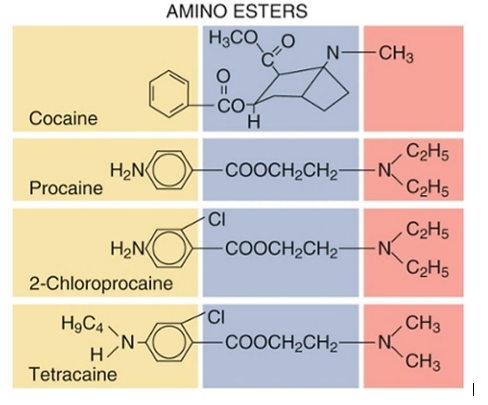
Amino Esters
- “Esters” contain an ester link between the aromatic end and the intermediate chain.
- The ester family includes cocaine (the first local anesthetic used clinically), Procaine (Novocaine), 2-Chloroprocaine (Nesacaine), and Tetracaine (Pontocaine).
- Esters are metabolized by plasma cholinesterase, with the exception of cocaine which is metabolized by hepatic carboxylesterase.
- Patients with atypical plasma cholinesterase have increased risk of toxicity from esters due to reduced or absent plasma hydrolysis.
- Para-aminobenzoic acid (PABA), a metabolite of esters, has been implicated in allergic reactions in humans.
- Patients with hepatic impairment or increased BUN, those receiving some chemotherapeutic drugs, and expectant mothers may have decreased levels of circulating plasma cholinesterase.
- Remember the one-eyed “ester” bunny (all esters have one “i” in their name).

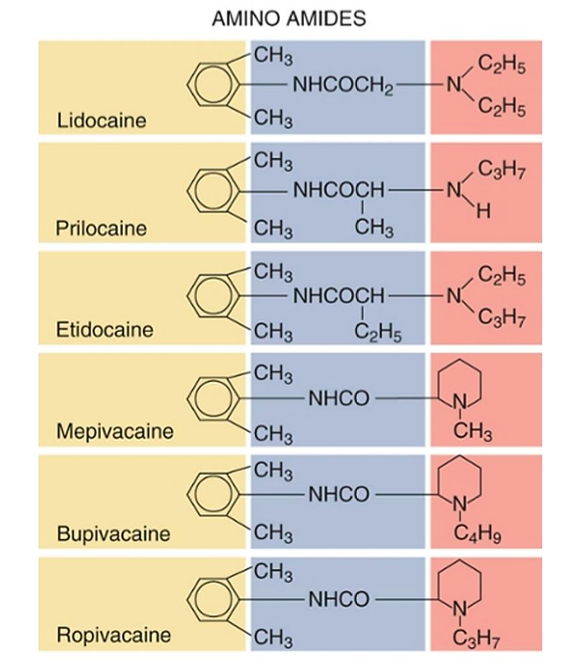
Amino Amides
- “Amides” possess an amide link between the aromatic ring end and the tertiary amine end.
- The amide family includes Lidocaine (Xylocaine) (the first amide local anesthetic), Ropivacaine (Naropin), Bupivacaine (Marcaine), L-bupivacaine, Prilocaine (Citanest, EMLA), etidocaine, and Mepivacaine (Carbocaine).
- Amides are metabolized by hepatic cytochrome P450-linked enzymes, with the exception of articaine, which is inactivated by cleavage of a methyl ester on the aromatic ring by plasma carboxylesterase.
- Amides are not metabolized to PABA and thus have extremely low rates of allergic reactions.
- There is no cross-sensitivity between the ester and amide classes of local anesthetics; however, there may be an allergic sensitivity to a preservative and metabolite common to both classes (methylparaben resembles PABA).
Potency, Duration, and Lipid Solubility
- Increased lipid solubility correlates with increased penetration of the neural membrane by local anesthetic.
- Lipid solubility is often expressed as a partition coefficient, which compares the solubility of a local anesthetic in a nonpolar solvent (lipid) like octanol with an aqueous, polarized solvent such as water.
- The size of the alkyl substituents on the aromatic ring, on or near the tertiary amine of the local anesthetic molecule, determines the lipophilicity (lipid solubility, or the molecule’s attraction to membrane lipids, AKA hydrophobicity) and subsequently the potency (and to some extent the duration of action and time to onset) of the local anesthetics.
- Thus, increasing the size of the alkyl substituent increases the potency and partially accounts for the lengthened duration of action of a local anesthetic by increasing its lipophilicity/ hydrophobicity.
- In short, increasing the lipid solubility of a local anesthetic increases its potency, lowers the toxic threshold limits, and increases the duration of action of the drug.
- When local anesthetic drugs of increasing potency are utilized in regional anesthesia, a lower concentration of local anesthetic has the same efficacy as less potent drugs at higher concentrations, as the dose-response curve shift to the left. Higher potent local anesthetics also have much lower toxicity dosing limits, requiring a lower concentration of anesthetic to be utilized in regional anesthesia.
- Utilizing lower concentrations of highly potent, lipid soluble local anesthetics will result in a slower onset of the drug due to a reduced concentration gradient diffusing into the axonal membrane.
- Example: Bupivacaine (Marcaine) 0.5% (surgical strength) has a slow onset, long duration of action; whereas, Lidocaine (Xylocaine) 2% (surgical strength) has a fast onset, short duration of action.
Duration of Action
- Besides lipid solubility, duration of action is also affected by the vasoconstrictive or vasodilatory effects of each particular local anesthetic and the vascularity of compartment which influences redistribution from the site of injection and uptake into the central circulation.
- Duration of action also correlates with a local anesthetic’s protein binding/lipid solubility characteristics. The greater the protein binding/lipid solubility, the longer local anesthetics remain tightly bound to neural tissue/proteins and thus are less susceptible to reuptake, redistribution, and metabolism/elimination.
- There is a direct relationship between the dose of local anesthetic given and the duration of blockade. Dose= [Concentration] x [Volume]. Studies have shown that the concentration of a local anesthetic has a greater effect on overall duration for peripheral regional blocks than the absolute volume administered, especially in a relatively low vascularized neural compartment.
- Adjuvants can increase the duration of action when administered concomitantly with local anesthetics.
- The duration of a block can be extended nearly indefinitely by the addition of a catheter-based technique.
- In summary, to increase the duration of action of local anesthetics, use a drug that has intrinsic vasoconstrictive properties, select a drug with a high lipid solubility/potency, give an adequate dose, use an adjuvant, and/or place a catheter for long-term continuous infusions.
pH and pKa
- As a reminder, ALL local anesthetics are weak BASES.
- Local anesthetics can alternate between their ionized (cationic, charged, protonated, or polar) and non-ionized (anionic, neutral, non-protonated, non-polar) forms.
- Local anesthetics MUST penetrate the neuron’s lipid membrane in order to block the inactivated sodium channel. Local anesthetics can ONLY penetrate the lipid membrane in their NON-ionized form. Therefore, local anesthetics are ONLY effective in their NON-ionized form.
- A local anesthetic’s pKa is the pH at which 50% of the local anesthetic is in the ionized form and 50% is in the NON-ionized form.
- Therefore, the pH of the liquid or tissue that the local anesthetic is injected into determines how much of the ionized and non-ionized forms exist in a local anesthetic solution.
- Lidocaine (Xylocaine) has a pKa of 7.8, so if placed in a solution with a pH of 7.8, Lidocaine (Xylocaine) will exist in equal parts (50/50) ionized and non-ionized forms.
- This explains why sodium bicarbonate (HCO3-) can be added to local anesthetics in order to speed onset by driving more of the local anesthetics into the nonionized form while making the compartment injected into a more alkaline environment.
Chirality
- Bupivacaine (Marcaine) is a racemic (equal amounts of the dextrorotatory and levorotatory forms) mixture of (R)- and (S)-stereoisomers.
- Ropivacaine (Naropin) and levobupivacaine (L-bupivacaine or Chirocaine) are single enantiomers (mirror images) of the (S)-stereoisomer of bupivacaine which were developed in response to the severe cardiotoxicity of bupivacaine (50/50 mix of the (R) and (S) enantiomers).
Comparative Attributes of Esters
- Cocaine’s attribute of vasoconstriction is unique among local anesthetics and makes it particularly suited to ENT applications.
- Most local anesthetics demonstrate a biphasic vasoconstriction at lower dosages and vasodilation with increasing dosages, with the exception of cocaine which causes vasoconstriction at all times due to the inhibition of uptake of norepinephrine by presynaptic neurons and thus potentiating neurogenic vasoconstriction.
- Although an ester, cocaine is metabolized by both plasma and hepatic esterases.
- One study demonstrated no difference in the anesthetic or vasoconstrictive effects of a Lidocaine (Xylocaine) and oxymetazoline mixture compared to cocaine.
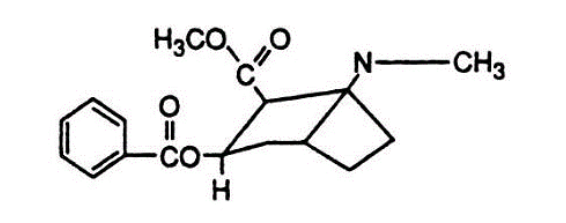
Cocaine
- Procaine (Novocaine) is typically used in dentistry. It was the first injectable local anesthetic and was used extensively until the proliferation of Lidocaine (Xylocaine). SAB with 10% procaine has a high incidence of precipitating nausea which lasts < 1 hour.
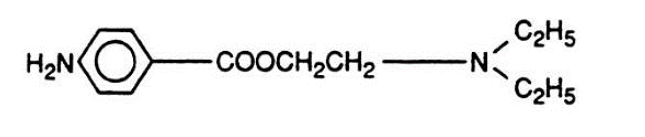
Procaine (Novocaine)
- Chloroprocaine (Nesacaine) has a notably short duration of action (<1 hour) and rapid onset of action. Epidural use of 3% 2-Chloroprocaine (Nesacaine) is associated with a subsequently reduced effectiveness of epidurally administered opioids and Bupivacaine (Marcaine) (possibly due to the low pH of Chloroprocaine (Nesacaine) decreasing the non-ionized fraction of Bupivacaine (Marcaine)
- The FDA has recently approved Chlorotekal (chloroprocaine), a preservative-free drug, to be administered in the intrathecal space and has gained popularity in short, outpatient procedures.
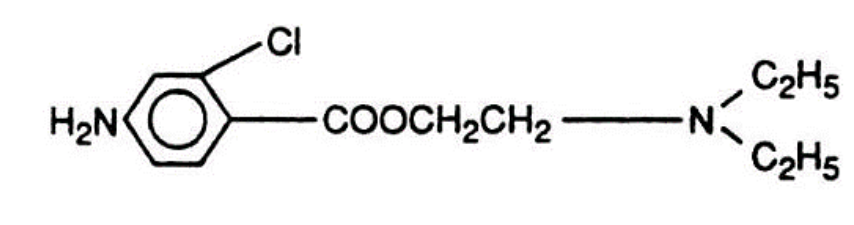
Chloroprocaine (Nesacaine)
- Tetracaine (Pontocaine) is often used topically on the eye or can be administered in SAB with an extended duration of action (1.5-2.5 hours), and when combined with epinephrine can last up to 4 hours; however, this combination possesses the undesirable risk of precipitating transient neurologic symptoms. As CSF does not contain plasma cholinesterase, an intrathecal injection of Tetracaine (Pontocaine) will produce a neuraxial blockade until the drug has absorbed into the systemic circulation. Although a member of the ester class, Tetracaine (Pontocaine) is metabolized significantly slower than its ester relatives.
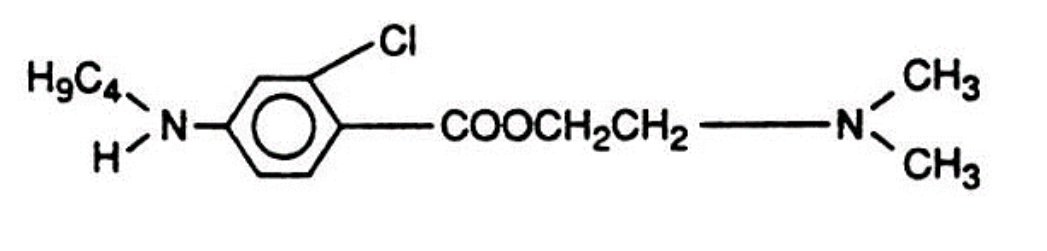
Tetracaine (Pontocaine)
- Benzocaine (Lanacaine, Cetacaine, Hurricaine) is the only local anesthetic that is a weak acid (pKa 5), so at physiologic pH, the drug is almost completely non-ionized, results in an extremely rapid onset of about 30 seconds or less and is especially useful for anesthetizing the mucous membranes of the oropharynx prior to performing EGD, bronchoscopy, or TEE procedures. Cetacaine contains 14% benzocaine, 2% Tetracaine (Pontocaine), and 2% butamben, while Hurricane is 20% benzocaine. Exceeding the recommended dosage of 300 mg can precipitate methemoglobinemic reaction.
Comparative Attributes of Amides
- Lidocaine (Xylocaine) is the most widely used local anesthetic in the world and possesses antiarrhythmic properties. Lidocaine (Xylocaine) metabolism parallels hepatic blood flow. Concerns regarding TNS (transient neurologic symptoms) and cauda equine syndrome have prompted some reservations of using Lidocaine (Xylocaine) for SAB. Textbooks recommend using less than 60-75 mg of 5% in dextrose for SAB as well as avoiding continuous SAB infusion, especially with the use spinal microcatheters.
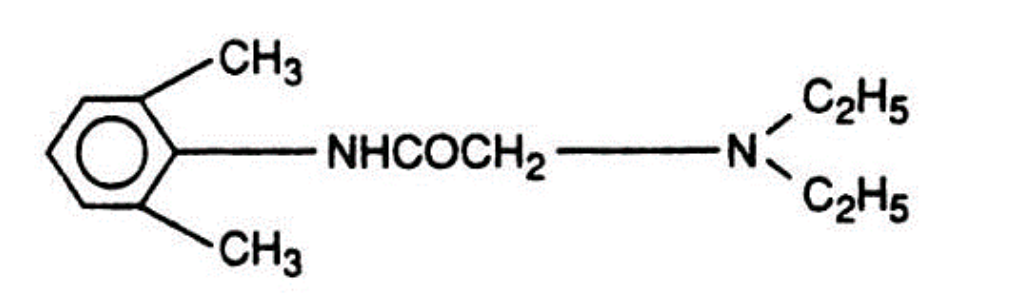
Lidocaine (Xylocaine)
- Prilocaine (Citanest, EMLA) is a constituent of EMLA (eutectic mixture of local anesthetics) cream. When metabolized, Prilocaine (Citanest, EMLA) can produce both ortho-toluidine and nitro-toluidine, of which ortho-toluidine, an oxidizing compound, converts hemoglobin to methemoglobin (in doses > 600 mg) more quickly than methemoglobin can be reduced to hemoglobin. Methemoglobin has the inability to bind and transport oxygen or carbon dioxide and presents clinically as cyanosis and hypercarbia. Methemoglobinemia can be treated with 1-2 mg/kg IV methylene blue (max dose of 8mg/kg) given over 5 minutes but may take 20-60 minutes for resolution of symptoms.
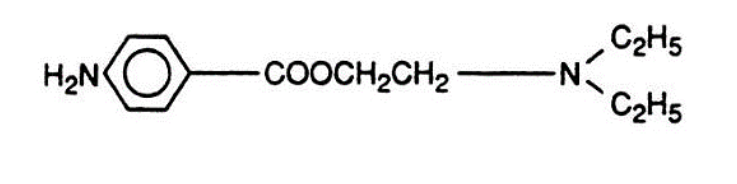
Prilocaine (Citanest, EMLA)
- Mepivacaine (Carbocaine) is occasionally mixed with Bupivacaine (Marcaine) to hasten the onset of peripheral nerve block or can be used in a SAB for approximate duration of 1.5 hours (40 mg), Admixing of a long and short local anesthetic has demonstrated in studies to produce minimal decreases in onset times and has shown to reduce the duration of a block. Mepivacaine (Carbocaine) features an anesthetic profile similar to Lidocaine (Xylocaine) but with a slightly longer duration and decreased effectiveness as a topical agent.
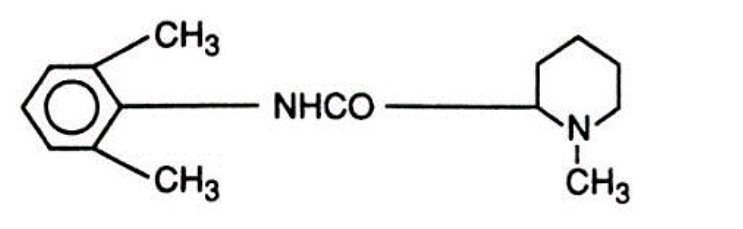
Mepivacaine (Carbocaine)
- Dibucaine is the most slowly eliminated of all the amides. It is better known for its ability to inhibit the actions of plasma cholinesterase (AKA butyrylcholinesterase) by over 70%, whereas atypical plasma cholinesterase is inhibited only about 20% by dibucaine. Patients suspected of having atypical plasma cholinesterase [which is associated with a significantly extended duration of drugs cleaved by the enzyme such as succinylcholine and Chloroprocaine (Nesacain)] can have their degree of enzyme suppression assayed using dibucaine, giving them a “dibucaine number”.
- Bupivacaine (Marcaine) has great versatility but is associated with significant cardiotoxicity when inadvertently injected intravascularly.
- Bupivacaine (Marcaine) is frequently used for infiltration, peripheral nerve block, SAB, and epidural anesthetics.
- Sensory block can be accomplished with lower concentrations of the drug, while a motor block requires higher concentrations to achieve surgical strength anesthesia.
- Bupivacaine (Marcaine) dampens the rapid phase of depolarization in Purkinje fibers and ventricular muscle tissue greater than Lidocaine (Xylocaine). Papillary muscles treated with Bupivacaine (Marcaine) also recover more slowly from a “use-dependent block” than papillary muscles treated with Lidocaine (Xylocaine).
- Pregnancy induces an increased sensitivity to the cardiotoxic effects of Bupivacaine (Marcaine).
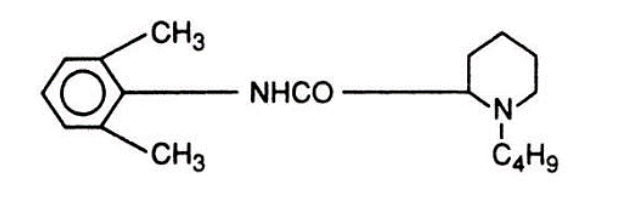
Bupivacaine (Marcaine)
- Ropivacaine (Naropin) is a single enantiomer of the (S)-stereoisomer of Bupivacaine (Marcaine).
- Compared to Bupivacaine (Marcaine), Ropivacaine (Naropin) demonstrates faster sodium channel recovery after eliciting a cardiac action potential, produces less negative inotropy on isolated cardiac tissue (decreased cardiotoxicity), and is less potent (1:1.3 to 1:1.5) than Bupivacaine (Marcaine).
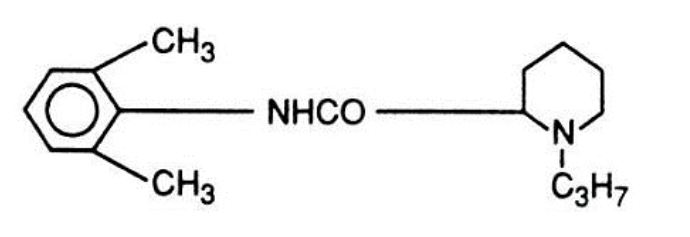
Ropivacaine (Naropin)
- Levobupivacaine (Chirocaine) is the single S (-) enantiomer of Bupivacaine (Marcaine).
- Similar in characteristics to Ropivacaine (Naropin) in demonstrating an improved cardiotoxic profile when compared to Bupivacaine (Marcaine).
- Not available in the US
- DepoBupivacaine (Exparel) is bupivacaine encapsulated in microscopic, multi-vesicular liposomes containing many small spheres of bupivacaine separated by lipid membranes.
- By incorporating liposomal technology, the duration of action can be extended up to 72 hours.
- FDA has approved Exparel for interscalene, TAP, and infiltrative blocks.
- Cannot be mixed with any local anesthetic other than bupivacaine in a ratio 1:2 (plain bupivacaine: Exparel). Failure to follow FDA labeling guidelines may cause an immediate release of the liposome-encased bupivacaine and resultant toxicity.
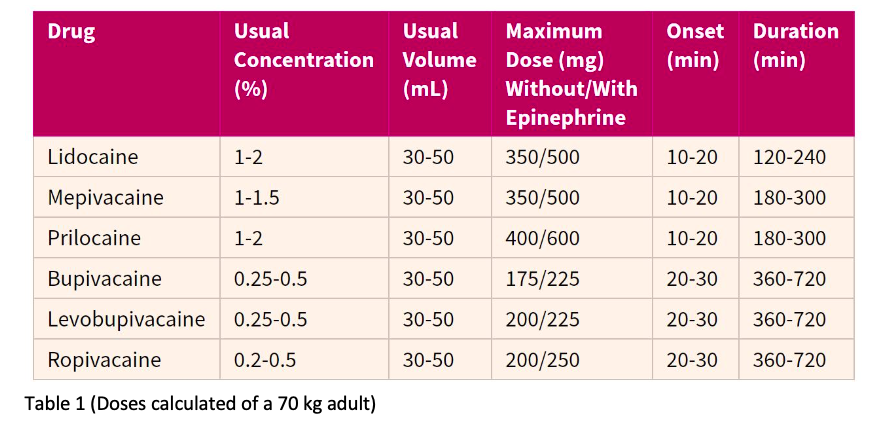
Dosing Parameters and Toxicity Considerations
- Dosage recommendations vary amongst reference texts, are not evidence-based, and cannot account for variations amongst patients and practices.
- As dosage is increased, so is the probability of local anesthetic systemic toxicity from inadvertent intravascular injection or absorption.
- As toxicity is presumed to be additive, DO NOT use the maximum doses of two separate local anesthetics in combination to calculate a toxic dose.
- Ex: If a 50/50 mix of Lidocaine (Xylocaine) and Bupivacaine (Marcaine) is being administered concomitantly, the toxic dose for each of these drugs would be 50% of their published toxic dose (See Table 1).
- Ultrasound guided blocks require less local anesthetic volume than conventional nerve stimulator-guided blocks due to the ability in precisely determining needle-to-nerve placement proximity and “real time” recognition of the distribution area of the local anesthetic injected around a plexus and/or a nerve.
Local Anesthetic Adjuvants
Vasoconstrictors
- The addition of 5 micrograms of epinephrine per milliliter (mcg/ml) of local anesthetic (1:200,000) reduces systemic absorption and prolongs the duration of a regional block.
- Epinephrine’s effect on alpha adrenergic receptors may contribute to a small degree of analgesia.
- Avoid using epinephrine in locations lacking collateral vessels (fingers, nose, toes, ear).
- Extremely useful in reducing vascular uptake in fascial plane compartment blocks and extending the duration of analgesia.
- Useful as an intravascular marker (1:400,000) in large volume, interfascial plane blocks as an assessment indicator of an early LAST event.
- Phenylephrine (2-5 mg) may be added to SAB block to extend the duration of a block but should be avoided in peripheral regional anesthesia.
Steroids
- Dexamethasone is the most commonly used steroidal adjuvant as it significantly extends the duration of blockade. The exact mechanism is unknown but is thought to be related to inhibition of phospholipase A2 and activation of glucocorticoid receptors.
Alpha-2 agonists
- Dexmedetomidine and Clonidine increase the duration of both motor and sensory block by hyperpolarizing the neuronal membrane through a alpha-2 adrenergic mediated mechanism.
- Clonidine may be added to epidural and intrathecal administrations to activate endogenous analgesic mechanisms arising from alpha-2 adrenergic receptors.
Opioid mixed agonist/antagonists
- Buprenorphine can extend the duration of block by several hours but can result in an increased incidence of PONV.
NMDA agonists
- Ketamine prolongs the action of local anesthetics, but has adverse effects and is not recommended as a adjuvant in regional anesthesia.
Sodium Bicarbonate
- Decreases onset by 3-5 minutes by shifting local anesthetic more into the nonionized form and altering the pH of the injected environment.
Pharmacokinectics of Local Anesthetic Systemic Toxicity (LAST)
- Plasma concentrations of local anesthetics are dependent on the total dosage injected, the site of injection, the rate of redistribution, the rate of metabolism and excretion, and patient-dependent factors, i.e. age, hepatic function, pregnancy, and nutritional status (affects protein binding), and if an adjuvant such as epinephrine was included.
Absorption
- Absorption is greatest for intercostal nerve blocks and caudal anesthesia, moderate for lumbar epidural anesthesia, and least after brachial plexus blocks and subcutaneous infiltration.
- A mnemonic for remembering the sites of absorption from greatest to least is:
- In = intravenous
- Time = tracheal
- I = intercostal
- Can = caudal
- Please = paracervical
- Everyone = epidural
- But = brachial plexus
- Sister = subarachnoid, sciatic
- Sally = subcutaneous
Distribution
- There is a positive correlation between the rate of perfusion of an organ and its concentration of local anesthetic.
- Lung tissue extracts some local anesthetics rapidly [namely Lidocaine (Xylocaine), Bupivacaine (Marcaine), and Prilocaine (Citanest, EMLA)], so systemic concentration decreases as local anesthetics pass through the pulmonary circulation.
Metabolism and Excretion
- Esters undergo hydrolysis by pseudocholinesterase enzymes.
- Amides undergo hepatic cytochrome P450-linked enzymatic degradation.
- Because of their undeveloped hepatic enzyme systems, neonates and infants exhibit prolonged elimination of amides.
- Neonatal toxicity with Lidocaine (Xylocaine) is related to the accumulation of its major metabolite, monoethylglycinexylidide.
- Patients with liver disease and congestive heart failure exhibit a markedly extended half-life clearance of Lidocaine (Xylocaine).
Local Anesthetic Systemic Toxicity (LAST)
- An elevated plasma concentration of local anesthetic can cause deleterious, toxic side effects.
- LAST occurs in approximately 1 out of every 1000 blocks.
- LAST can present immediately due to intravascular injection or can have a delayed presentation due to redistribution/absorption of local anesthetic from highly vascular tissue.
- Pathophysiology of local anesthetic systemic toxicity (LAST) related to the central nervous system:
- CNS signs of toxicity can occur before the cardiovascular system becomes unstable if the drug is absorbed in a linear fashion.
- Cardiac side effects are encountered with higher plasma concentrations of local anesthetics, and CNS symptoms may never be apparent.
- One source suggests that the rate of local anesthetic plasma concentration increase may be more important than the total dose in eliciting CNS toxicity.
- The pathophysiology of CNS toxicity reflects several proposed processes:
- The initial excitation phase possibly results from blockade of inhibitory pathways in the cerebral cortex or could be the result of unopposed release of the excitatory neurotransmitter glutamate.
- Even greater plasma levels of local anesthetic suppress both inhibitory and excitatory circuits, leading to profound CNS depression.
- This state of CNS depression is exacerbated by a concomitant respiratory acidosis produced from respiratory depression and eventual respiratory failure as elevated PaCO2 dilates cerebral vasculature, increasing cerebral blood flow and causing even greater amounts of local anesthetic to be delivered to the brain in a positive feedback loop.
- Additionally, the increased concentration gradient of extracellular CO2 drives plasma CO2 into neuronal cells, leading to decreased intracellular pH. This state of intracellular acidosis accelerates the change of local anesthetic from base form to cationic form, which cannot exit through the lipid bilayer of the cell membrane due to polarization, leading to ion trapping of local anesthetic intracellularly and further perpetuating CNS toxicity.
- Finally, plasma protein binding (α1-acid glycoprotein and albumin) with local anesthetics is decreased in respiratory depression and acidosis, further increasing the amount of unbound local anesthetic available in systemic circulation and worsening the condition of a LAST event. In a normal physiologic pH, plasma proteins help bind local anesthetics, reducing overall plasma concentrations of local anesthetics.
- It is imperative to adequately ventilate the patient experiencing LAST event in order to avoid or prevent the acidic, protein unbinding of local anesthetics.
- Pathophysiology of local anesthetic systemic toxicity (LAST) related to the cardiovascular system:
- Local anesthetics affect the cardiovascular system directly as well as indirectly via sympathetic and parasympathetic efferent blockade.
- Profound hypotension can result from the relaxation of vascular smooth muscle in the arterial system.
- Local anesthetics slow the rate of depolarization in the Purkinje fibers and ventricular muscle tissue by blocking fast sodium channels, as evidenced by a lengthening PR interval and QRS complex.
- Very high doses of local anesthetic dampen pacemaker activity in the sinus node causing bradycardia or sinus arrest.
- Dose-dependent inotropic depression is attributed to negative modulation of calcium release from the sarcoplasmic reticulum and inhibition of sarcolemmal sodium and calcium channels.
- Hypercapnia, acidosis, and hypoxia increase the negative inotropic and chronotropic actions of local anesthetics, and increase the frequency of arrhythmias.
- Beta blockers, calcium channel blockers, and digoxin decrease the threshold for cardiac toxicity.
- Signs and Symptoms of LAST
- CNS signs and symptoms of LAST begin with dizziness, lightheadedness, facial numbness or tingling, or circumoral numbness and progress to auditory and visual disturbances such as ringing in the ears or slurred speech. Other patients may exhibit drowsiness or disorientation.
- As plasma levels of local anesthetics increase, CNS toxicity manifests as tremors, twitching, or shivering. This may be followed by tonic-clonic seizures that originate from either the amygdala or temporal lobes.
- Even greater plasma levels of local anesthetics produce CNS depression, wherein seizure activity ceases and the respiratory drive is ablated, culminating in respiratory arrest. This state of CNS depression may be seen without a preceding period of excitation if CNS depressant medications have been administered prior to the local anesthetic insult.
- Cardiovascular signs and symptoms of LAST include hypotension, bradycardia, tachycardia, various arrhythmias including ventricular tachycardia and fibrillation, and eventual arrest. These cardiac symptoms may be appear with CNS prodromal signs or seizures if a rapid cardiotoxic dose of local anesthetic is absorbed.
Treatment of LAST
- Intralipid 1.5 ml/kg
- Lipid emulsion possibly provides a “lipid sink” or a separate intravascular compartment for lipophilic drugs, such as local anesthetics, to be extracted from aqueous plasma, thus decreasing the systemic concentration. Local anesthetics bound to lipid molecules are then transported and delivered to muscle, adipose tissue, and other noncardiac tissue until elimination of local anesthetic can be metabolized by the liver (amides) or esters (plasma esterases) over a longer period of time.
- Lipids also have a positive inotropic effect on cardiac calcium channels improving contractility of the cardiac muscle.
Prevention of LAST
- Aspirate gently to prevent a false-negative result that can be caused by the inner lumen of a vessel adhering to the needle orifice.
- Aspirate frequently after every 5 milliliters (ml) of local anesthetic that is injected.
- Monitor the ECG for early changes in QRS morphology, rate, rhythm, or ectopy.
- Observe for anaechoic spread of local anesthetic on the ultrasound display. If this is not witnessed during injection, local anesthetic is probably being injected into a vessel.
References
- Berde CB, Strichartz GR. Local anesthetics. In: Miller RD, ed. Miller’s Anesthesia. Philadelphia, PA: Elsevier; 2015:1029-1054.
- American Society of Regional Anesthesia and Pain Medicine. Checklist for treatment of local anesthetic systemic toxicity. https://www.asra.com/advisory-guidelines/article/3/checklist-for-treatment-of-local-anesthetic-systemic-toxicity. Updated 2017. Accessed February 11, 2017.
- Horlocker TT, Kopp SL, Wedel DJ. Peripheral nerve blocks. In: Miller RD, ed. Miller’s Anesthesia. Philadelphia, PA: Elsevier; 2015:1721-1752.
- Drasner K. Local anesthetics. In: Miller RD, Pardo MC, eds. Basics Of Philadelphia, PA: Elsevier; 2011:130-142.
- Maheshwari K, Naguib MA. Local Anesthetics. In: Flood P, Rathmell JP, Shafer S, eds. Stoelting’s Pharmacology and Physiology in Anesthetic Practice. Philadelphia, PA: Wolters Kluwer; 2015:282-313.
- Maheshwari K, Brown DL, Mounir-Soliman L, Sites BD, Spence BC. Pharmacology and ultrasound. In: Farag E, Mounir-Soliman L, eds. Brown’s Atlas Of Regional Philadelphia, PA: Elsevier; 2017:3-14.